Antimicrobial Nanoemulsion
Antimicrobial nanoemulsions are oil-in-water droplets that range from 200-600 nm. They are composed of oil and water and are stabilized by surfactants and alcohol. They are manufactured from ingredients which are on the Generally Recognized as Safe (GRAS) list. Active ingredients are approved for over-the-counter human applications. A high-energy state is formed in the particle using a high-speed mixer. The active ingredient and the high energy are essential for the antimicrobial mechanism of action. Additional reduction of size is achieved by a high-pressure microfluidizer. This additional reduction of size results in more energy units per volume. Additional ingredients are added to enhance the nanoemulsion spectrum of activity or to improve its stability. The concentrated (neat) emulsions are diluted 10- to 100-fold in water, resulting in a stable final product. The dilute emulsions are milky in consistency and appearance; additional thickeners could be added to increase its viscosity and prevent running in specific applications.
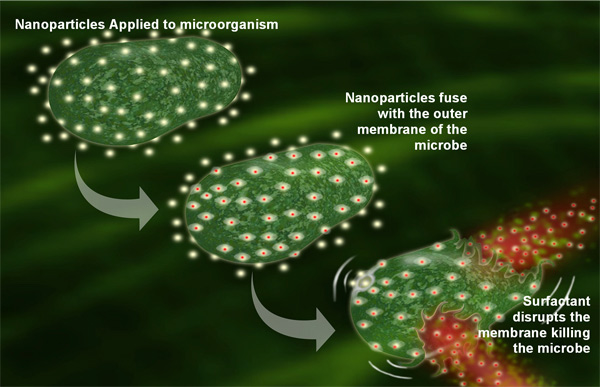
The nanoemulsion has a broad spectrum activity against bacteria (e.g., E. coli, Salmonella, S. aureus), enveloped viruses (e.g., HIV, Herpes simplex), fungi (e.g., Candida, Dermatophytes), and spores (e.g., anthrax). The nanoemulsion particles are thermodynamically driven to fuse with lipid-containing organisms. This fusion is enhanced by the electrostatic attraction between the cationic charge of the emulsion and the anionic charge on the pathogen. When enough nanoparticles fuse with the pathogens, they release part of the energy trapped within the emulsion. Both the active ingredient and the energy released destabilize the pathogen lipid membrane, resulting in cell lysis and death (Fig. 1). In the case of spores, additional germination enhancers are incorporated into the emulsion. Once initiation of germination takes place, the germinating spores become susceptible to the antimicrobial action of the nanoemulsion (Fig. 2).
Dilute emulsions showed stability when stored at 40°C for over 1 year and at room temperature for over 3 years. They can also withstand several cycles of heating and cooling. This would be enough to have viable marketable products.
When Bacillus spores contaminate a mucosal membrane, nutritional factors present in the damaged tissue and serum serve as germination enhancers. These germination enhancers initiate the germination of the bacillus spores and the tough outer shells of the spores then become vulnerable to the lethal effect of the nanoemulsion. This will result in disruption of the spore.
A unique aspect of the nanoemulsions is their selective toxicity to microbes at concentrations that are non-irritating to skin or mucous membrane. This safety has been tested in several animal species and verified during human clinical trials. The safety margin of the nanoemulsion is due to the low level of detergent in each droplet, yet when acting in concert, these droplets have sufficient energy and surfactant to destabilize the targeted microbes without damaging healthy cells. As a result, the nanoemulsion can achieve a level of topical antimicrobial activity that has only been previously achieved by systemic antibiotics. As a result, the FDA approved proceeding directly to phase II clinical trials for topical treatment of Herpes Labialis. The study, which has just been concluded, involved 332 subjects who received the drug. The results will be available at 3Q'05. There have been no safety issues and the product has been well tolerated. Other applications to follow include onychomycosis, genital herpes, herpes zoster, and vaginitis.
Laboratory results with mice indicate that treatment of their nares with nanoemulsion prior to exposure to pathogens offers protection against pathogen challenge. The emulsion also acts as a mucosal adjuvant by presenting the pathogen to the immune system, which can induce protective immunity in the absence of an active infection. In contrast, the presentation of other forms of inactivated pathogens to mucosal surfaces does not yield an effective immune response, suggesting that the nanoemulsion-killed organisms are uniquely immunogenic. This would prove useful in prevention of respiratory infections, including influenza.
Another valuable application of nanoemulsion is in the field of bioterrorism and includes decontamination of humans, surfaces and buildings following biological bioattacks. Recently, the formulation has been enhanced to achieve killing for B. anthracis in 45-60 minutes.
NanoBio Corp., a spin-off company from the University of Michigan, has an exclusive license to develop and commercialize the antimicrobial emulsion for topical and mucosal applications.