Targeting Chemotherapeutic Drugs to Prostate Cancer Cells
Using Dendrimers Coupled to Anti-PSMA Antibody
This Department of Defense (DOD)-U.S. Army Medical Research Acquisition Activity-funded project seeks to develop targeting mechanisms for anti-cancer drugs directed towards PSMA-bearing cells that can subsequently be coupled to imaging and signal detection platforms. Through other grant support (NIH-NCI), we have developed a dendrimer-based platform for specifically targeting drugs, imaging agents and radiopharmaceutics to breast, head, and neck cancers. We will extend these studies and test anti-PSMA-dendrimer-drug conjugates for targeting of prostate cancer cells in tissue culture. We will investigate the ability to target anti-PSMA antibody conjugates to prostate cancer cells in vivo and evaluate the ability of anti-cancer chemotherapeutic agents coupled to these conjugates to specifically kill prostate cancer cells in vivo.
Dr. James R. Baker is the Principal Investigator on this project. Dr. Anil Patri is a synthetic organic chemist who has worked on the dendrimer design and construction and surface and internal modification of dendrimers, as well as on the loading of the dendrimers with humanized antibody to prostate-specific membrane antigen (PSMAext). They collaborate with Dr. Kenneth J. Pienta, who has extensive expertise in the area of prostate cancer, as well as Dr. Neil H. Bander from the New York Presbyterian Hospital and Cornell University Medical Center, who has worked on antibody-based targeting technology.
Prostrate cancer is a serious and insidious disease. While improved screening techniques and hormone manipulation therapy have resulted in early diagnosis of prostate cancer and effective treatment for many men, this disease still impacts the male population significantly, accounting for approximately 38 percent of all deaths of men over the age of 50.
Importantly, despite the advances in diagnosis, there are real problems involved in therapeutic decisions. Early diagnosis has not clarified the benefits of aggressive therapy, such as radical prostatectomy, because the outcomes of this therapy in localized disease are not always curative. In particular, some patients with localized tumors will have progressive disease despite having prostate ablative therapy. The reasons for this are unclear but probably relate to differences in the cancer phenotype and the inability to identify minimally metastatic disease outside of the prostate at the time of prostatectomy.
Despite this, most men do well until their prostate cancer becomes resistant to hormonal therapy. When this occurs, there are few options because most chemotherapeutic protocols are ineffective and the disease is difficult to monitor. In particular, widely metastatic, hormone-resistant prostate cancer has an extremely poor prognosis. Thus, new therapeutic initiatives that would better characterize the extent of the disease and aid in the treatment of hormone-non-responsive prostate cancer would be extremely helpful.
Targeted Cancer Therapy
Targeted therapy is potentially an important means of attacking cancer. Better outcomes for hormone- resistant tumors may be achieved by specifically delivering drugs, radionuclides, or toxins to tumors to specifically kill tumor cells without affecting surrounding tissue. This is an attractive option for prostate cancer that is poorly responsive to standard therapy, particularly when the sensitivity of the cancer to an anticancer agent is similar to that of normal tissue. In addition, many types of cancer can infiltrate throughout normal tissue or bone and therefore cannot always be adequately addressed with external radiotherapy.
This disease could also benefit from the targeting of radiopharmaceuticals or other agents specifically to the cancer cells. Targeted therapy is dependent on a specific marker for the cancer cells that can be identified with affinity agents such as antibodies (for antigens) or ligands (for receptors). Also, the coupling of imaging reagents to targeting agents can specifically identify cancer cells in a variety of tissues with greater sensitivity than traditional imaging.
Targeting in Prostate Cancer
In prostate cancer, the ability to target widely metastatic, hormone-resistant disease would be the most sought-after advance. This is because the control of non-hormone-responsive metastatic lesions would allow most men to live a nearly full life span and die for reasons other than prostate cancer since local disease rarely causes death. However, prostate cancer at this stage is resistant to most chemotherapeutic agents. Therefore, it would be of minimal help to merely target a drug to deliver higher concentrations to cells.
There may be advantages if the therapeutic could deliver the drug to specific parts of the cell, or maintain it within the cell to overcome the effects of multidrug resistance channels. Also, combinations of agents may yield synergistic anti-tumor effects. Targeted therapy with combinations of chemotherapeutic drugs may provide unique benefits in this regard because combinations of chemotherapeutic agents have shown utility in treating hormone-resistant prostate cancer, but this application is limited by toxicity.
Coupling dendrimers to these agents would not only target the drugs but could also solubilize some of these agents so they can be given intravenously. Additionally, the ability to target prostate cancer with combinations of radioisotopes and radio-sensitizing drugs could yield real benefits.
The combination of targeted therapeutics with targeted high-resolution imaging agents is also of potential interest. Current techniques of targeted imaging agents, using radio-scintillography, do not provide high-resolution images. Nonetheless, these techniques have been able to identify disseminated disease better than traditional imaging such as MRI or CT scanning. It is even possible that individuals considering local prostate ablative therapy or having metastatic disease could have therapeutic decisions modified on the basis of the finding of distant disease or could have adjunctive therapy that might lead to more durable response. Thus, targeted combination drug therapy for prostate cancer in conjunction with targeted imaging could yield true advances in care.
Targets in Prostate Cancer
Targeting agents associated with the prostate have been identified and characterized. At least two major prostate antigens have been identified: prostate specific antigen (PSA) and prostate specific membrane antigen (PSMA). PSA was the first identified prostate antigen and has been remarkably useful in screening for this disease. However, there are several issues about using this as a target for therapy. First, it is secreted and present in high concentrations (micrograms/ml) in the serum. This could block targeting to tumor cells before the therapeutic or imaging agent can bind or enter a cancer cell. In addition, PSA appears to be less useful for targeting and is expressed at lower levels in hormone-resistant cancer. As a result, the ability to target hormone-non-responsive cancer, one of the major goals of targeting therapy, is less likely to be effective using PSA. In contrast, PSMA appears to offer theoretical benefits to targeted therapy and imaging.
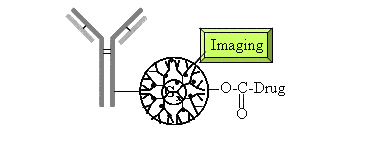
PSMA is expressed by most prostate cancers and may also be expressed in the vasculature of prostate tumors. It is expressed at very high levels in most cancers, and it appears that expression of this antigen is maintained or may increase in hormone-resistant tumors or with hormone manipulation. Importantly, the levels of this antigen in serum are approximately 100-fold lower than that of PSA, suggesting that it would not be as likely to block targeting. 99Tc-labeled antibodies to PSMA have been able to identify prostate metastasis through imaging studies, and further characterization of the capability to identify metastasis may be achievable with higher-resolution imaging techniques. In addition, examination of lymph nodes of individuals undergoing radical prostatectomy suggests that the presence of PSMA (as detected by messenger RNA) is highly predictive of recurrence post-prostatectomy. Imaging approaches, however, are limited because of the inability to couple most agents other than radionucleotides directly to antibodies. Taken together, these findings suggest that PSMA may be a useful antigen to target therapy and imaging of prostate cancer. Therefore, the identification of metastatic disease and treatment of hormone-resistant prostate cancer are areas that potentially benefit from targeted therapy.
Technical Approach for Target Therapy: Smart, Nanomolecular Therapeutics
Given the requirements for designing a device small enough to efficiently enter cells but able to perform multiple smart tasks, the only currently available technology that serves these purposes is a nano-device. These are designed synthetic materials with structures less than 10 to 100 nanometers in size. Useful devices are limited to less than one hundred nanometers in diameter since this is the largest-sized particle that escapes the vasculature and that most cells will readily internalize. Unlike "nanomachines" based on fictional mechanical machines that have been "shrunken" to nanometer dimensions, several true molecular nano-structures have now been synthesized and used for drug delivery, gene transfer, antimicrobial therapeutics and immunodiagnostics. The Center at the University of Michigan is dedicated to the development of these devices and has been supported by NCI to develop them for breast and head and neck cancer and will adapt this approach to prostate cancer applications.
"Antibody-dendrimer conjugates for targeted prostate cancer therapy" Anil K.Patri, Thommey Thomas, James R. Baker Jr., Neil H. Bander. Polym. Mater. Sci. Eng., 2002, 86, 130.
"Dendritic Polymer Macromolecular Carriers for Drug Delivery" Anil K. Patri, István J. Majoros, James R. Baker Jr., Curr. Opin. Chem. Biol. 2002, 6, 466.