Cancer Cell Targeted Drug Delivery — In Vitro
The goal of the biological research in our laboratories is to achieve efficient, tissue-specific homing of therapeutic agents into cancer cells. This is crucial in cancer therapy in order to completely eradicate all neoplastic cells and to prevent the regrowth of cancerous tumors. Such a "targeted" therapy has the potential to decrease the potent systemic toxicity caused by currently practiced therapy that is potentially limited by a narrow chemotherapeutic index. To achieve this goal, we conduct biological studies using dendrimer/based "nanodevices" that contain the necessary basic components, such as a targeting agent that would specifically bind to the tumor, a fluorescent molecule for tracking its presence in cells, and a drug to kill the targeted cells. Dendrimers are branched complex polymers which are commercially available in different "generations" having different molecular weights and sizes. They have recently emerged as one of the most suitable drug carriers because of their biocompatible properties and their nanometer size, dimension, and structural architecture, which mimic certain biomolecules. Dendrimer-based, targeted drug delivery is founded on the principle that if a receptor is expressed specifically or in excess on the surface of a cancer cell, the dendrimer carrying a drug and a ligand for the receptor travels through the circulation, binds specifically to the cancerous cells, and delivers the drug to induce programmed cell death. Conjugation of several molecules of a targeting agent onto the dendrimer will result in an increase in the dendrimer's avidity for binding to the targeted cells through multivalent interaction because of the binding of multiple targeting molecules to their receptor.
We are currently testing a variety of receptors for small molecules, proteins, and antibodies which are relatively more specific, or overexpressed, in cancer cells as potential targeting sites. Folic acid receptor (FAR) is overexpressed on the surface of a variety of malignancies, such as cancer of the head and neck and of the ovary. KB cells which express high FAR are suitable in vitro models for investigating the binding of FA-conjugated dendrimers. Polyamidoamine (PAMAM) dendrimers with FA as the targeting molecule and drugs such as methotrexate and taxol as the chemotherapeutic agents are able to target FAR-expressing KB cells in vitro (Thomas et al., J Medicinal Chemistry. 48: 3729-3735, 2005; Majoros et al., J Medicinal Chemistry, in press) and in "Mice xenograft KB tumors in vivo" (Kukowska et al., Cancer Research 65: 5317-5324, 2005).
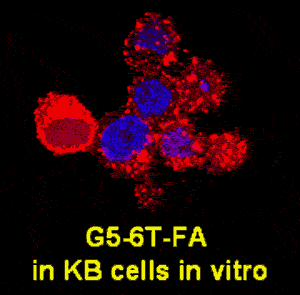
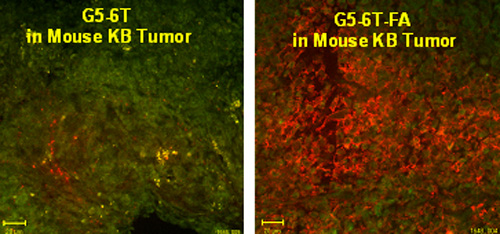
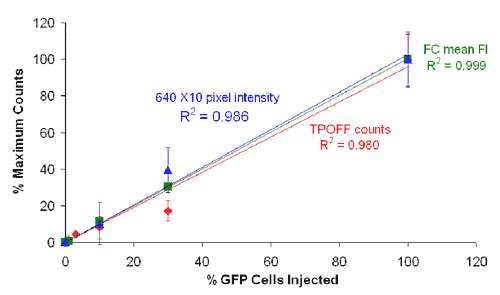
We have demonstrated the applicability of dendrimers as platforms for antibody- and peptide-based targeting of tumor cells (Thomas et al., Biomacromolecules 5: 2269-2274, 2004; Patri et al.; Bioconjugate Chem. 15: 1174-1181, 2004; Shukla et al.). Our studies have also shown for the first time the applicability of a targeted dendrimer nanodevice to monitor a subcellular function such as the mitochondrial membrane potential (Sassanella et al., Biomacromolecules, submitted). The studies we have done in collaboration with Drs. Theodore Norris and Jingyong Ye at the Center for Ultrafast Optical Science have shown the potential application of a two-photon optical fiber device for monitoring the levels of a fluorescently labeled dendrimer in tumors in mice (Thomas et al., Biophysical Journal 86: 3959-3965, 2004).
We have also shown the applicability of a "tectodendrimer" cluster generated by covalently linking 4-5 G5-PAMAM "shell" dendrimer molecules carrying folic acid to a single "core" G5-PAMAM dendrimer carrying the dye FITC to target FAR-expressing KB cells. In addition, dendrimer clusters can be synthesized by linking two dendrimer molecules through the self-assembly (annealing) of two single-stranded complementary DNA molecules covalently conjugated onto two separate dendrimers carrying two different functions. Our studies have proven the biological applicability of such a synthesized cluster dendrimer with FA and FITC as the two functions on the two dendrimer molecules (Choi et al., Chemistry and Biology 12: 1-9, 2005).
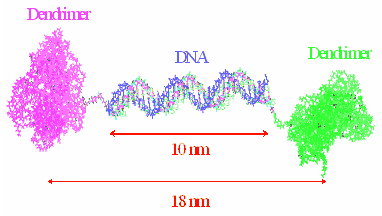
The DNA-linked dendrimer platform allows the combinatorial synthesis of conjugates containing multiple functions for tumor analysis such as imaging, drug delivery, and cancer cell-killing.
In summary, our recent studies have shown that the PAMAM dendrimers which can be synthesized in large scale under GMP guidelines are biocompatible macromolecule carriers suitable for the targeted delivery of drugs into tumor cells in vitro and in vivo. Multiple molecules such as a tumor-specific targeting agent, a chemotherapeutic drug, and an apoptosis-detecting agent can be covalently conjugated on its surface. This "smart" engineered multifunctional dendrimer nanodevice mimics a biological molecule and performs multiple biological tasks, such as binding to a cancer cell, releasing a drug to cause the death of the cancer cell, and measuring the extent of cell death. A "cluster" dendrimer conjugate may serve as a useful and versatile nanodevice with separate functions on separate dendrimer molecules. Such a nanodevice can be custom-synthesized for patient-specific needs by easily linking of different functional dendrimers through a short DNA molecule.