Imaging
We are developing MRI and SQUID imaging using targeted magnetic nanoparticle contrast agents. The contrast enhancement for the imaging is provided by superparamagnetic nanoparticles approximately 6 nm in diameter. Internalization of the contrast agent is through the high-affinity folate receptor, which is overexpressed in a variety of epithelial cancers. After introduction of the contrast agent, MR imaging is then performed. Similarly, detection of the superparamagnetic particles both in vitro and in vivo is being performed to determine the ultimate limits of the SQUID imaging technique. This approach allows the imagining of pre-clinical lesions and paves the way for intracellular delivery of functional imaging agents.
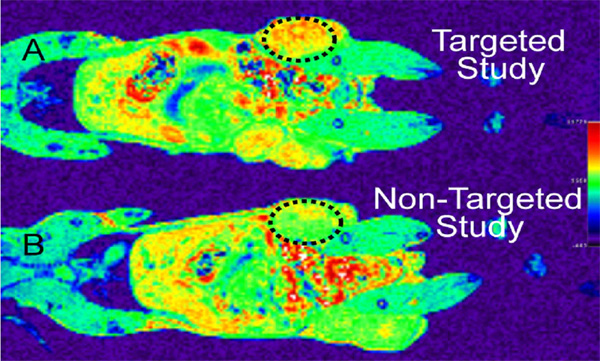
We have refined two synthetic approaches for the formation of targeted magnetic nanoparticles. The first method uses folic acid for targeting and Gd chelated within DOTA as the magnetic particle. These two components are conjugated to a dendrimer scaffold to form the targeted contrast agent. We have performed initial in vivo NMR studies of this material in murine models. The second synthetic approach involves the formation of a 6-nm FeO nanoparticle and then solubilization by exchange of the surfactant. This process has been completed and concentrated aqueous suspensions of the nanoparticles have been produced and analyzed.
In addition to the NMR, we are developing SQUID techniques for detection of the magnetic particles. In agarose samples, we are able to detect the presence of 10 ng of magnetic particles. We have begun placing the nanoparticles into mice to examine the sensitivity in vivo. We will continue to explore the use of magnetic detection of the contrast agent via SQUID magnetometry.
We are conducting toxicity and loading studies in vitro and will be able to determine both loading of the cells and toxicity. in vivo mouse studies and determination of targeting efficacy will follow.