Dendrimers
The Michigan Nanotechnology Institute for Medicine and Biological Sciences (M-NIMBS) is developing bio-active nanodevices based on dendrimeric architecture. The Institute is constructing biologically active nanodevices from dendrimers. Nanotechnology is a new field, and these are the first working biologically active nanodevices. These nanodevices are undergoing tests to determine their operating parameters and biological activity. Currently the Institute is testing nanodevices as smart therapeutics for cancer. The Institute is building a catalog of components, each of which individually performs a particular biological task and each of which can be combined with others to produce a vast array of nanodevices.
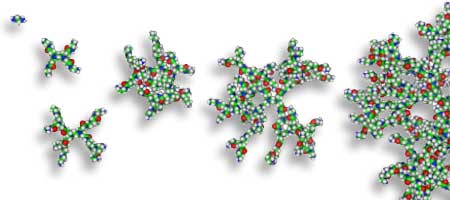
Dendrimers are spherical polymeric molecules. Dendrimers and proteins share several characteristics:
- Size (See figure 2)
- Weight
- Well-defined chemical structure where every atom and its bonds to other atoms is known
- Difficulty in determining the 3-dimensional position of each atom, yet a consistent, specific 3-dimensional structure exists
- Difficulty in performing chemical analysis
- Ease of cellular uptake
Dendrimers and proteins differ in that proteins are polymers made from 20 different monomers, while dendrimers are polymers made from two monomers: acrylic acid and a diamine. Dendrimers are polymers, as are all plastics. Nylon is a very well-known polymer that is made from a diacid and a diamine. Nylon and dendrimers share a very similar chemical heritage and structure, yet nylon differs in a most important way from dendrimers. Nylon and all plastics have very poorly defined chemical structures. These traditional polymers are mixtures of many different molecules, tangled together to give an average molecular structure and constant bulk properties. The distribution of the constituent molecules is probabilistic. In contrast, dendrimers are precisely defined chemical structures; all of the chemical bonds between the atoms can be accurately described. It is this consistency of structure that makes dendrimers the ideal (and to date the only) building block for creating a biologically active nano-material.
Using dendrimers, the Michigan Nanotechnology Institute for Medicine and Biological Sciences has demonstrated several biologically active nano-materials. The goal of the Institute is to create a catalog of nano-components which can be used as building blocks in the creation of a vast array of larger, more sophisticated nanodevices, which the Institute names Tecto-Dendrimers.
Structure
Dendrimers consist of a series of chemical shells built on a small core molecule. Each shell consists of two chemicals, always in the same order. This is much like a layer cake, where each layer consists of a cake-frosting pair. With dendrimers, each shell is called a generation and we can speak of G2.5, which, in our cake analogy, would consist of cake-frosting-cake-frosting-cake, with no frosting on the top cake layer.
Dendrimers are very much like ordinary organic molecules for the first three generations. They are small and floppy without much consistent or specific three-dimensional structure. By G4 they are beginning to become spherical and to take on a preferred three dimensional structure. By G5 they have a consistent and specific three dimensional structure. Beyond G5 they are highly structured spheres.
The surface of all full generations consists of multiple amines, while the surface of the half generations consists of multiple acids. These two kinds of surfaces provide the means of attachment of multiple different functional components.
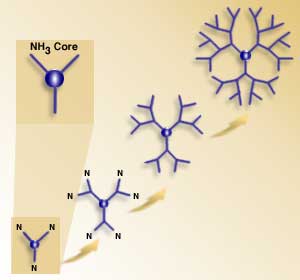
Dendrimers are branching molecules with the branching beginning at the core. Depending on the core, the dendrimer can start with 3 to 8 (or more) branches, with 3 and 4 being the most common number. Starting from the core, the dendrimer consists of long chains of atoms with a branch point about every half dozen atoms. At each branch point, the current chain of atoms becomes two chains of atoms. The molecular structure has the form of a tree with a great number of branches. The name "dendrimer" is derived from the ancient Greek word "dendron" (tree), and from the Greek suffix "-mer" (segment).
back to topComposition
Dendrimers consist of a core molecule and alternating layers of two monomers. Each pair of monomer layers completes a shell and a generation. The core generally consists of an amine core, although sugars and other molecules can be used. All core molecules share the characteristic of having multiple reaction sites that are identical. Even the simplest core possible, ammonia (NH3) has three amine reaction sites.
The core is mixed with an excess of the first monomer molecule which reacts with all of the core's reaction sites, giving rise to the first branches. This monomer molecule has two distinct reactive groups, one at each end. After one kind of end reacts, the other end will provide reaction sites for the next layer of the shell.
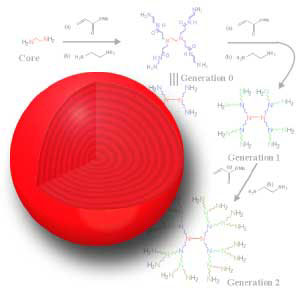
An excess of the second monomer, again a molecule which has two distinct reactive groups, one at each end, is reacted with this first layer to give the second layer and complete the first shell and the first generation. Each unreacted outer end of the second monomer provides a reaction site which can react with multiple molecules. This provides the branching and the reactions sites for the next shell.
Most of the dendrimers that the Michigan Nanotechnology Institute for Medicine and Biological Sciences synthesizes are polyamido molecules. At the outer surface of every full generation, the reactive groups are all amines. At the interface of the two layers making up the one shell of a generation, there is an amide linkage.
back to topSynthesis
There are a large number of molecules which can be used as cores, and an even larger number of possible monomers are available for building the layers. As with many plastics, particularly Nylon, this creates a great number of possibilities.
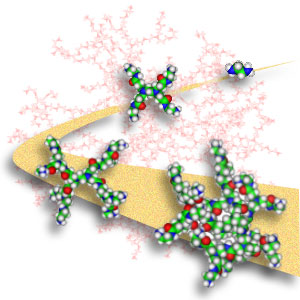
The canonical dendrimer starts with an ammonia core. This core is reacted with the double bond in acrylic acid to produce a tri-acid molecule. This tri-acid is reacted with ethylene diamine to produce a tri-amine (G0). This tri-amine is reacted with acrylic acid to produce a hexa-acid, doubling the number of acids in this half-generation (G0.5). Next, another round of ethylene diamine is reacted with the G0.5 to give a G1 molecule with six amines, twice the number at G0. This alternation of acrylic acid with ethylene diamine continues until the desired generation is reached.
back to topFunction
The Institute has completed testing of functioning biologic nano-devices based on dendrimers. These tests have been conducted in vitro on living cells. In August 2000, we began tests in vivo. The in vivo tests are to confirm that the nano-devices will work as therapeutic agents.
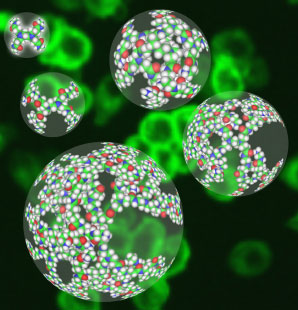
The Institute is conducting tests of these dendrimers as components of nano-devices. The Center is building a library of dendrimeric components from which a combinatory number of nanodevices can be made. Our focus is on nanodevices which are smart therapeutics in human disease. The library will contain components which will perform these tasks:
- Diseased cell recognition
- Diagnosis of disease state
- Drug delivery
- Reporting location
- Reporting outcome of therapy